UDI For Medical Device Software (MDSW) Under EU MDR - Decomplix
 decomplix.com
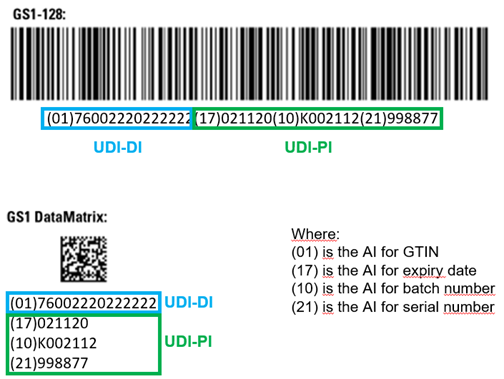
decomplix.com udi mdr gs1 datamatrix mdsw pis represented barcodes
Flyktninger På Tur Til Skjervøy - Skjervøy Kommune
UDI, FDA GUDID, HL7 SPL Submission, UDI For Medical Devices – Freyr
 freyrsoutions.wordpress.com
freyrsoutions.wordpress.com udi fda medical compliance devices timeline class hl7 submission spl healthcare device gs1 source timelines regulatory freyr ii
UDI (@Utlendingsdir) | Twitter
 twitter.com
twitter.com UDI Beginners Guide: Unique Device Identification (EU MDR And IVDR)
 easymedicaldevice.com
easymedicaldevice.com udi device unique mdr identification eu ivdr medical di barcode identifier basic date beginners guide information batch
Guarantee Form For Visits Garantiskjema For Besøk - UDI
 www.yumpu.com
www.yumpu.com Withdrawal Of Application Letter: Fill Out & Sign Online | DocHub
 www.dochub.com
www.dochub.com UDI Beginners Guide: Unique Device Identification (EU MDR And IVDR)
 easymedicaldevice.com
easymedicaldevice.com udi identification device ivdr infographic eu mdr unique
Skjema Til Bruk Ved Registrering Av Navn (PDF) - UDI Regelverk
 www.yumpu.com
www.yumpu.com Fullmakt Skjema Nav - Skjema
 skjema-1.blogspot.com
skjema-1.blogspot.com UDI & EUDAMED Explained Under EU MDR - Clinical, Regulatory
 www.ddismart.com
www.ddismart.com udi mdr eudamed labeling
Skjema For Arbeidstilbod - UDI
 www.yumpu.com
www.yumpu.com Udi, fda gudid, hl7 spl submission, udi for medical devices – freyr. Udi identification device ivdr infographic eu mdr unique. Udi (@utlendingsdir)

Catat Ulasan
Catat Ulasan